Kehamilan yang disertai diabetes gestasional, diabetes melitus tipe 1 ataupun tipe 2 yang sudah ada sebelumnya, memiliki risiko komplikasi yang lebih tinggi dibandingkan dengan kehamilan pada populasi umum. Komplikasi ini meliputi keguguran, persalinan prematur, preeklamsia, kematian perinatal, dan kelainan kongenital.¹
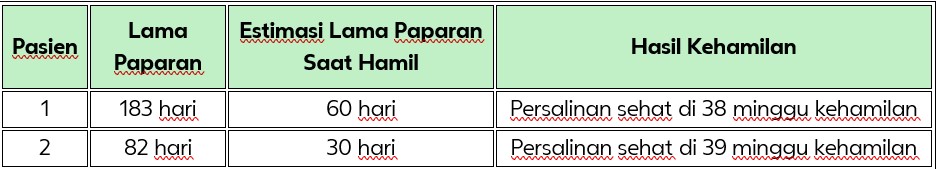
Insulin glulisine adalah analog insulin kerja cepat yang diindikasikan untuk pengobatan pada orang dewasa, remaja, dan anak-anak berusia 8 tahun ke atas dengan diabetes melitus yang memerlukan terapi insulin.² Selama uji klinis, dilaporkan terdapat dua kehamilan yang terpapar insulin glulisine. Tidak ditemukan malformasi atau komplikasi pada kedua kehamilan tersebut. Hasil dari kehamilan ini ditunjukkan pada Tabel 1. Penggunaan glulisine selama kehamilan tidak dikontraindikasikan, namun belum ada rekomendasi spesifik untuk populasi ini.
Tabel 1. Ringkasan Kehamilan pada Wanita yang Terpapar Glulisine
Doder, et al.,¹ menganalisis dan merangkum penggunaan insulin glulisine pada kehamilan dari berbagai sumber, termasuk dari data farmakovigilans dan uji klinis milik Sanofi, serta hasil dari pencarian literatur terkini dan komprehensif. Secara kumulatif, terdapat 303 laporan paparan kehamilan terhadap insulin glulisine. Dari jumlah tersebut terdapat 116 kelahiran hidup,12 keguguran spontan, 2 kematian janin intrauterin pada usia kehamilan lebih dari 28 minggu, 3 aborsi elektif serta 170 kasus tanpa informasi mengenai hasil kehamilan. Terdapat 6 kasus malformasi kongenital; 5 di antaranya disertai kelahiran hidup, sedangkan 1 kasus tidak diketahui status kelahirannya. Sampai saat ini, tidak ditemukan pola spesifik pada malformasi kongenital yang dilaporkan. Kesimpulannya, data yang ada hingga saat ini tidak menunjukkan adanya hubungan kausal antara penggunaan insulin glulisine dengan peningkatan risiko komplikasi kehamilan atau malformasi kongenital.
Tabel 2. Hasil Kehamilan dari 303 Paparan (Kasus Kumulatif hingga 30 Juni 2014)
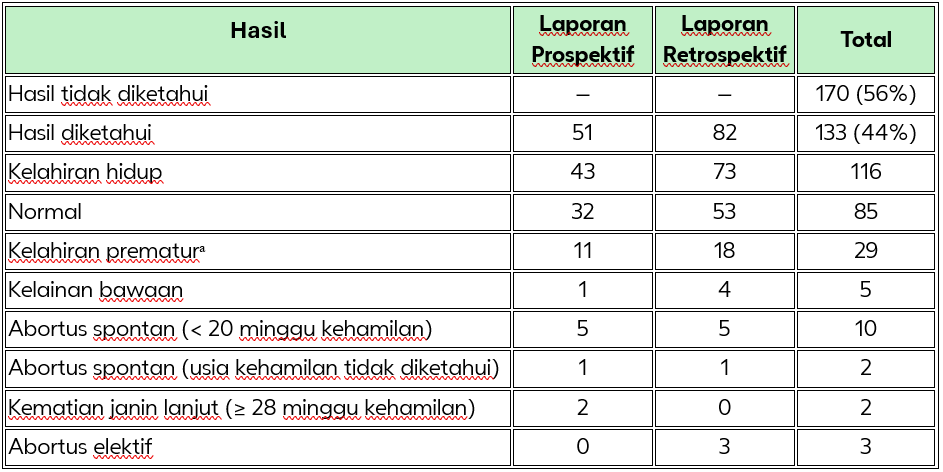
ᵃ Tiga kasus kelahiran prematur juga memiliki kelainan bawaan.
Kesimpulan:
Belum tersedia data yang memadai mengenai penggunaan insulin glulisine pada wanita hamil. Data terbatas (kurang dari 300 laporan hasil kehamilan) pada wanita hamil yang terpapar insulin glulisine setelah produk dipasarkan tidak menunjukkan adanya masalah keamanan baik terhadap ibu, janin, maupun bayi baru lahir. Penggunaan glulisine selama kehamilan tidak dikontraindikasikan, namun belum ada rekomendasi spesifik untuk penggunaannya pada populasi ini. Hati-hati saat meresepkan obat ini untuk wanita hamil. Pemantauan kadar glukosa darah secara ketat sangat penting. Pasien dengan diabetes gestasional atau diabetes yang sudah ada sebelumnya harus menjaga kontrol metabolik yang baik selama kehamilan. Kebutuhan insulin dapat menurun pada trimester pertama, namun umumnya meningkat pada trimester kedua dan ketiga. Setelah melahirkan, kebutuhan insulin biasanya menurun dengan cepat. Pasien dengan diabetes harus memberitahukan kepada dokter mereka apabila sedang hamil atau berencana untuk hamil. Belum diketahui apakah insulin glulisine diekskresikan ke dalam ASI manusia, namun secara umum insulin tidak masuk ke dalam ASI dalam jumlah yang signifikan dan tidak diserap secara sistemik melalui pemberian oral. Ibu menyusui mungkin memerlukan penyesuaian dosis insulin dan pola makan.
Gambar: Ilustrasi (downloaded from https://www.freepik.com/free-photo/young-latin-woman-pregnant-measuring-glucose-home_49701274.htm#fromView=search&page=1&position=0&uuid=4e051b01-78a7-4daa-bcdf-09589e47e929&query=pregnant+woman+with+diabetes)
Referensi:
- Doder Z, Vanechanos D, Oster M, Landgraf W, Lin S. Insulin Glulisine in Pregnancy - Experience from Clinical Trials and Post-marketing Surveillance. Eur Endocrinol. 2015;11(1):17–20. doi:10.17925/EE.2015.11.01.17
- Apidra® Informasi Produk Lokal Indonesia 2023
Untuk pertanyaan lebih lanjut mengenai informasi ini, silakan hubungi: indonesia.medicalinformation@kalventis.com
Apabila Anda mengetahui kejadian efek samping yang berkaitan dengan produk Kalventis, harap laporkan ke: farmakovigilans@kalventis.com
Informasi ini Hanya untuk Tenaga Kesehatan
ID-API-2025-09-KZWE (09/25)